Main Topics
Licensing
The licensing processes in our country are carried out according to the provisions of the Human Medical Products Licensing Regulation which was prepared in the scope of the No1262 Pharmaceutical and Medical Preparations Act and compliance with the European Union legislation and which went into force on 19.01.2005.
The application files are prepared and presented in CTD format in accordance with regulations. CTD is a format which has been established with the objective of making applications presented to the pharmaceutical licensing authorities in Europe, the U.S. and Japan organized and is a format that has been agreed on an international format.
CTD is comprised of five modules;
- Module 1: Administrative Information
- Module 2: Quality Information, Outside of Clinic and Clinic Summaries
- Module 3: Chemical, Pharmaceutical and Biological Information
- Module 4: Outside Clinic Reports
- Module 5: Clinical Study Reports
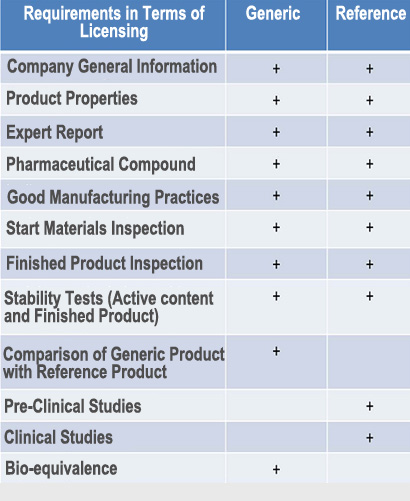
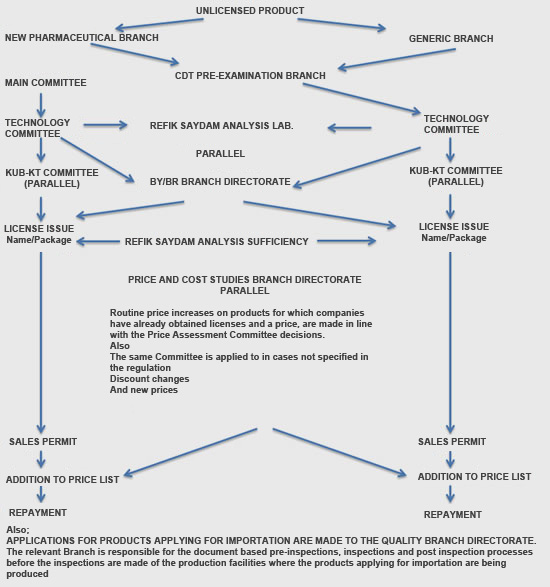
One of the problems being experienced in the licensing process is the 210 day product licensing period which in practice is much too long.
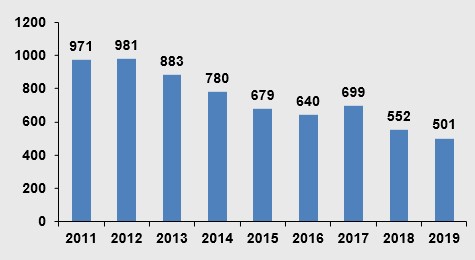
The number of drug licenses obtained over years
The number of licenses obtained in 2019 was 501.